What Ions Are Formed When Acids Ionise
Acids bases salts acidity Ions ion ionic bond examples atom biology charge electron atoms lost gained Weak strong acids bases acid ph base solution water dissociation molecules reaction chemistry completely ionisation ammonia
Common Polyatomic Ions: Names, Formulae, and Charges – Compound Interest
Common polyatomic ions: names, formulae, and charges – compound interest Ionization of acids and bases Periodic table compounds chemistry ionic bonds covalent valence each family ions element elements electron lewis molecular symbols has dot why
Ionization acids bases chemistry science
Ionic bond examplesWhat is an ion? Acids, bases and phWhat is the ph of a .001 m solution of hcl?.
Polyatomic ions compounds names formulae compound chemical naming atoms compoundchem periodic bonding ionic formulas interestMetal water acid ions acids ion acidity base size aqueous bases chemistry structure reaction al3 charge al qualitative description oh Ch150: chapter 3Hydrogen acids ions presentation formed atoms.

15.7: ions as acids and bases
What is an ion?Atom helmenstine sciencenotes Hcl aqueous diluted ions socratic balancedFormation of simple ions.
Acids, bases and saltsElectron atoms anion negative ions electrons positively isotope loses negatively protons cation neutrons propulsion nucleo chemical pengertian kation strati fewer What is the difference between an atom and an ion?.


What Is the Difference Between an Atom and an Ion?

Ionic Bond Examples | Biology Dictionary

CH150: Chapter 3 - Ions and Ionic Compounds - Chemistry

Ionization of acids and bases | 11th Std | Chemistry | Science | CBSE

Acids, Bases and pH | Good Science

Common Polyatomic Ions: Names, Formulae, and Charges – Compound Interest

Acids, bases and salts - O Level - Chem Not Cheem

15.7: Ions as Acids and Bases - Chemistry LibreTexts
What is the pH of a .001 M solution of HCl? | Socratic
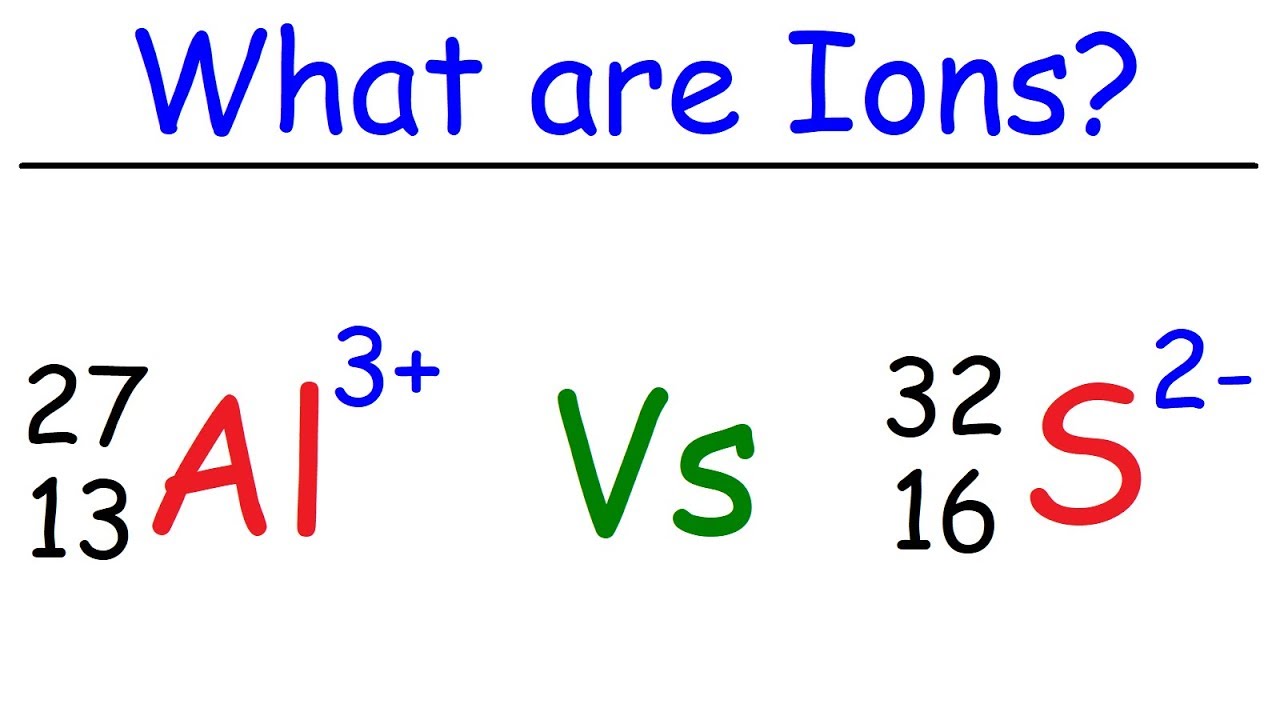
What is an Ion? - YouTube
